Developing Magnetic Particle Imaging (MPI) for studying brain function
Magnetic Particle Imaging (MPI) is a rapidly developing imaging modality that directly measures and maps the concentration of injected superparamagnetic iron oxide nanoparticles (SPIONs) [1], [2]. Similar to fMRI methods [3], [4], we propose using MPI to detect SPION concentration in the brain as a measure of cerebral blood volume changes (ΔCBV). We hypothesize that MPI will provide a significant sensitivity benefit compared to fMRI due to the large size of the SPION magnetization compared to nuclear spins and the detection efficiency of MPI.
Rodent ΔCBV changes detected through MPI
In MPI, the SPION magnetization is driven into saturation by an oscillating magnetic field at the drive frequency, f0. The generated odd-order harmonics are measured by Faraday receive coils. Usually a field-free point/line (FFP/FFL) is used to localize the signal.
Our initial work includes the development of single-sided magnetic particle (MP) detector for detecting and quantifying ΔCBV in rats during a hypercapnia challenge. The single-sided detector that relies on the localized sensitivity of the coils (~0.5 cm3 region). Figure 1 shows a schematic of our instrument, which uses a NI-DAQ-based console. The drive chain produces up to a 15mT drive field at the surface of the rodent’s skull using a Techron 7224 power amplifier, a high-power low-pass filter, and a 4.4cm dia. drive coil. The receive chain consists of a nested 1.7cm dia. gradiometer coil, a notch filter attenuating the drive frequency, and a low-noise preamplifier (SR560).
The system was used to record [Fe] changes in a 282g Sprague Dawley rat undergoing CBV modulation by hypercapnia. A dose of 8.7 mg [Fe] /kg of Ocean Nanotech SPP-25 particles was given as the SPION agent. The ventilation was alternated in 5 minute intervals between hyperventilation with normal air and mildly depressed ventilation rates with a 5% CO2 air mixture. Figure 2 shows the resulting 3rd harmonic MPI signal during 4 cycles of the hypercapnia paradigm in one rat. The trace was low-pass filtered to reduce physiological noise, and a linear baseline trend was also removed. A 10% signal modulation was measured with CNR = 50.
Human fMPI design analysis
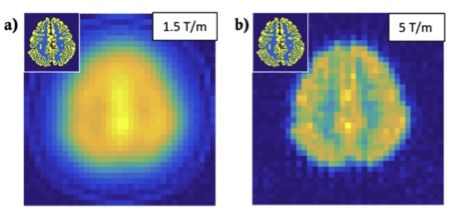
As human-sized MPI systems have not been attempted, we assessed the technical challenges of scaling MPI from rodent to human brain [5]. We developed a full-system MPI simulator to test arbitrary hardware designs and encoding practices, and examined tradeoffs imposed by constraints that arise when scaling to human size as well as safety constraints (PNS and central nervous system stimulation) not considered in animal scanners, thereby estimating spatial resolutions and sensitivities achievable with current technology. Using a projection FFL MPI system (Fig. 3), we examined coil hardware options and their implications for sensitivity and spatial resolution. Figure 4 shows a simulated fMPI FFL projection brain imaging of blood volume contrast using an FFL gradient of 1.5T/m and 5T/m. We estimate that an fMPI brain scanner is feasible, although with reduced sensitivity (20x) and spatial resolution (5x) compared to existing rodent systems. Nonetheless, it retains sufficient sensitivity and spatial resolution to make it an attractive future instrument for studying the human brain; additional technical innovations can result in further improvements.
[1] B. Gleich and J. Weizenecker, “Tomographic imaging using the nonlinear response of magnetic particles,” Nature, vol. 435, no. 7046, pp. 1214–1217, Jun. 2005.
[2] P. W. Goodwill et al., “X-Space MPI: Magnetic Nanoparticles for Safe Medical Imaging,” Adv. Mater., vol. 24, no. 28, pp. 3870–3877, 2012.
[3] J. B. Mandeville et al., “Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation,” Magn. Reson. Med., vol. 39, no. 4, pp. 615–624, Apr. 1998.
[4] D. Qiu, G. Zaharchuk, T. Christen, W. W. Ni, and M. E. Moseley, “Contrast-Enhanced Functional Blood Volume Imaging (CE-fBVI): Enhanced Sensitivity for Brain Activation in Humans using the Ultrasmall Superparamagnetic Iron Oxide Agent Ferumoxytol,” NeuroImage, vol. 62, no. 3,pp. 1726–1731, Sep. 2012.
[5] E. E. Mason, C. Z. Cooley, S. F. Cauley, M. A. Griswold, S. M. Conolly, and L. L. Wald, “Design analysis of an MPI human functional brain scanner,” Int. J. Magn. Part. Imaging, vol. 3, no. 1, Mar. 2017.
Acknowledgement: Research reported here was supported by the National Institute for Mental Health (NIMH) and the National Institute for Biomedical Imaging and Bioengineering under the Brain Initiative of the National Institutes of Health award number R24MH106053 and then U01EB025121.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.